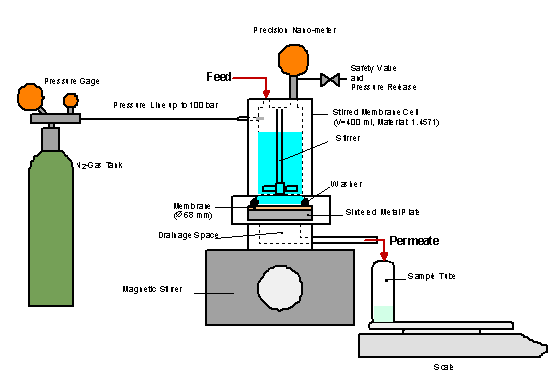
Figure 1. Stirred filter cell for
research on a laboratory scale
CLEANER
PRODUCTION MANAGEMENT FOR METAL WORKING INDUSTRIES AS EXAMPLE OF METAL PICKLING
WASTE-WATER
I.
GEHRKE, V.KEUTER
Fraunhofer-Institute
for Environmental, Safety and Energy Technology (UMSICHT),
Abstract: This article presents a general overview of cleaner
production (CP) methods for pickling processes. An example of a successfully
implemented membrane plant for recycling spent pickling liquor is given.
I. INTRODUCTION
From 1983 until now the steel production was
doubled leading to an enormous waste formation [6]. Along with growing world
population the need of steel is still increasing and thus the environmental
pollution caused by waste and waste-water from metallic working industries
become a more and more emerging problem. In many countries, recently, novel
stringent regulations and directives were introduced in order to both safe
water quality and force the metal industry to a more sustainable handling of
process water including new recycling strategies.
Against this background “Cleaner Production”
(CP) management leading to savings in resources and costs turns out more
attractive for the metal industry. The United Nations Environment Program
(UNEP) define CP as follows: "Cleaner
production means the continuous application of an integrated, preventative
environmental strategy to processes, products and services to increase
eco-efficiency and reduce risks to humans and the environment".
Cleaner Production focuses on minimising resource use and directly avoiding the
creation of pollutants, rather than implementing end-of-pipe technologies. It
involves rethinking products, processes and services to move towards
sustainable development.
The objectives of CP shall be reached by the
means of conserving raw materials and energy, eliminating toxic raw materials,
reducing quantity and toxicity of emissions and wastes, reduction of negative
impacts along the product life cycle and incorporation of environmental
concerns into designing and delivering services.
In addition to the environmental benefits also
economic returns are to be gained, e.g. improved production efficiency, lower
cost for waste disposal and waste-water treatment (WWT) as well as new and
improved market opportunities via better image.
There is existing a wide variety of cleaner
production methods for the diverse metal industry sectors including casting,
finishing and machining processes [2, 4].
Within this work CP waste-water management for
metal finishing applications, namely pickling processes was theoretically
investigated. Additionally, more practical a pilot plant for integrated
pickling bath recycling was developed and installed.
II. CP MANAGEMENT FOR PICKLING PROCESSES
1. Overview
Related to metal finishing a high amount of liquid
and solid waste containing toxic metals, alkaline and acidic solutions are
produced. Among others some of the most harmful components within metal
finishing waste are chromium, cyanide and concentrated acids. They are
accumulated in paint, inks, sludge, solvents, liquid waste and rinse water [3,
5, 8, 10, 13, 14].
Belonging to the category of liquid waste pickling
bathes are one of the most significant sources of highly contaminated acidic
process water.
Pickling is defined as the removing of inorganic
impurities from a metallic surface by the means of a liquid, causing chemical
solving processes and/or blasting of oxide coatings from the metallic surface
[1]. The pre-treated metallic work piece is turned over to subsequent
processing like galvanizing, painting or extrusion. Except for some additives
the pickling liquor mainly consists of mineral acids particularly hydrochloric
acids, sulphuric acid, nitric and hydrofluoric acids.
2. CP measures
Within the pickling process acid is consumed, water
is evaporated and metal salts are enriched. As a result a certain amount of
acids have to be continuously added to maintain free acid levels. However, if
the metals content of the solution exceeds a specific value (e.g. 50-60 g/l in
stainless steel pickling liquor) metal salts will start to crystallize leading
to the dumping and replacement of the bath [1]. Thus, the pickling process,
including subsequent process steps like rinsing, generates a considerable
quantity of spent pickle liquor comprising large amounts of nitrate, fluoride,
chloride, sulphate and metal salts. Spent pickling is considered as hazardous
waste by EPA. In former days, it was disposed of in landfills following lime
neutralization.
Nowadays advanced Cleaner Production strategies,
which aim to in-situ regeneration, are applied to the pickling process. Besides
this more elaborate and cost intensive techniques there are certain easy
handling CP methods listed below for reducing the quantity of liquid waste
within the pickling process [3, 10, 14].
• Careful and clean working
• Installation of drip trays and splash guards
• Drag out reduction through
- longer drainage times for batch treated work
pieces
- squeezing of continuously treated work pieces
• Improving rinsing through
- cascade rinsing (reuse of rinsing water)
- spray rinse of the work pieces above the pickling
bath
- air or mechanical agitation for better cleaning
efficiency
These measures are very helpful for reducing
waste-water from pickling processes. However, recovery/regeneration
applications are obsolete, since the quantity of generated waste-water
resulting form a bath change is much larger compared to drag out losses, etc.
The most important processes for the recycling of
pickling acids are pyrohydrolysis and retardation.
Pyrohydrolysis bases on the principal of thermal
decomposition. At high temperatures water and acid are evaporated, metal salts
are decomposed into metal oxide and are continuously removed. This process
route enables the total regeneration of both the free acid and the acid that
was chemically bounded to metals. The metal oxides are transferred to diverse
industries for reuse [1, 11].
In contrast to the total regeneration that is
reached by thermal process retardation is limited to the recycling of free
pickling acid. However, acid retardation is by far the most widely used system
due to its low investment costs and simplicity, reliability and superior
performance. Retardation of pickling bases on the selective adsorption and
desorption of acid on specific ion exchange resins. Because of the discharge of
acids bounded to the metals periodically a small quantitiy of fresh acid has to
be added [1, 11].
Another CP strategy
for a more environmental friendly and resource saving pickling process is the
complete or part substitution of the chemical pickling process through
mechanical pickling if suitable. Thus, surface impurities and oxide coatings
are removed by using certain abrasive materials (e.g. glass or steel ball,
carbide pats or rotating brushes) [14].
3. Case study
1. Situation: Due to more stringent legislations and the need for saving costs the
plant engineering sector has a growing interest in more environmental and
resource saving processes in the area of metal finishing. Among others they are
searching for an alternative more efficient process for the reuse of pickling
bathes.
Though nanofiltration
is an established membrane process for separation of metals, so far, only a few
research activities focus on the implementation of nanofiltration processes in
the recycling of pickling acids [9].
Thus, within our work
we investigated the behaviour of different nanofiltration (NF) membranes to
corrosive acidic conditions in order to develop and implement a novel
nanofiltration plant at pilot scale for recycling of pickling acids.
2. Materials and methods: First screening tests with different
nanofiltration membranes were conducted (Tab. 1).
Table
1. Nanofiltration membranes
for screening tests [7]
|
Membrane |
Material |
pH |
Tmax |
pmax |
|
NF 1 |
PES |
1-14 |
90°C |
40 bar |
|
NF 2 |
NN |
0-14 |
70°C |
35 bar |
|
NF 3 |
PES |
0-14 |
95°C |
40 bar |
|
NF 4 |
NN |
NN |
NN |
NN |
|
NF 5 |
NN |
NN |
NN |
NN |
The experiments were performed using a membrane test cell made of Hastelloy
steel (Fig. 1).

Figure 1. Stirred filter cell for
research on a laboratory scale
The operation pressure is applied
by a gas cushion and the mass transfer is realized by a magnetic stirrer. The
filter cell features a maximum pressure of 100 bar and a filling volume of
400 ml. Filter areas up to 36 mm² can be tested within the filter
cell.
The applied pickling acid was
provided from a large steel company and is characterized as follows (Tab. 2):
Table 2. Composition of the nitric/fluoric pickling
acid [7]
|
Parameter |
Wert |
|
pH |
< 1 |
|
Fluoride |
25.5 mg/l |
|
Nitrate |
7.4 g/l |
|
Chrome |
222 mg/l |
|
Iron |
1.2 g/l |
|
Molybdenum |
10 mg/l |
|
Nickel |
166 mg/l |
|
Titan |
0.75 mg/l |
The main parameters that have to
be determined during the screening tests are the filtrate flow and the
retention of the different components.
Nitrate was detected by ion chromatography. Fluoride was measured by the
means of an ion selective electrode. For the determination of metals an
ICP-spectrometer was applied. The filtrate flow was gravimetrically measured.
3. Results: Basing on a variety of experiments the membrane
screening of five different membrane types yields the following results (Tab.
3).
Though the NF2
features the best results the NF1 was preferred for subsequent experiments
since the NF1 showed a higher resistance within the acidic milieu. The
properties of the NF1 relating to the metal and nitrate retention are presented
below (Fig. 2). In order to determine the acidic stability the rejection was
measured before and after eight weeks storage of the membrane in the pickling
acid.
The metal
retention maintained relatively constant. The slight decrease of the rejection
was probably caused by oxidation processes. In contrast the nitrate retention
drastically changed from 75 % to -1.5 % which is very beneficial for
the separation of nitric acid from metal salts, since the nitrate was even
concentrated in the filtrate.
The
behaviour of the permeate flux is shown below (Fig. 3).
Table
3. Evaluation of the screened membranes for nitric/fluoric acidic
pickling:
+ positive, - negative, 0 indifferent [7]
|
Retention |
NF1 |
NF2 |
NF3 |
NF4 |
NF5 |
|
NO3- |
+ |
+ |
+ |
+ |
0 |
|
F+ |
0 |
0 |
0 |
- |
0 |
|
Cr- and Fe- |
+ |
+ |
+ |
+ |
+ |
|
Ni- and Ti- |
0 |
0 |
0 |
+ |
+ |
|
Mo |
- |
+ |
- |
+ |
+ |
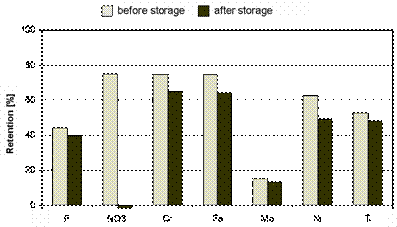
Figure 2. Metal and nitrate retention of NF1
before and after eight weeks storage
with nitric/fluoric acid pickling [7].
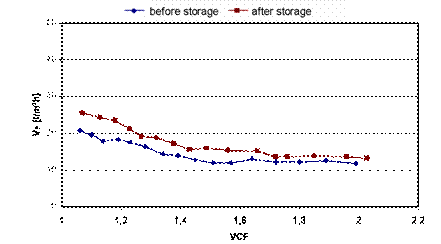
Figure
3. Permeate flux of NF1 versus VCF[1] before and after eight
weeks storage with nitric/fluoric acid pickling [7].
Despite the storage of the membrane in the pickling
acid just a slight increase of the permeate efficiency after storage was
recognized. Presumably, due to oxidation processes the pore structure of NF1
became more permeably. Although the permeate flux measured after storage
decreased along with rising VCF from 25 l/hm² to 15 l/hm², it
maintained relatively high compared to the other tested membranes featuring
permeate fluxes lower than 10 l/hm².
4. Implementation: Based
on the results gained within the membrane screening tests a nanofiltration
membrane plant at pilot scale for the recycling of pickling liquor was planned
and constructed for a large steel company, which is one of the world's leading
manufacturers of stainless flat products. It was implemented on site in a
pickling line where the plant ran in bypass operation (Fig. 4).
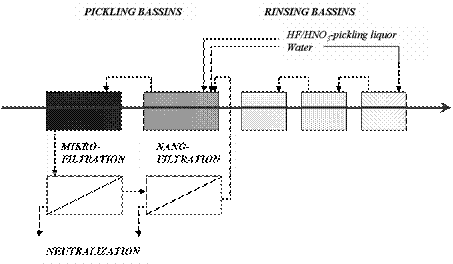
Figure 4. Implementation of the
nano-filtration membrane plant in the pickling line [11].
The process chain consists of the pickling step followed by a rinsing
process. Due to drag losses and evaporation water and fresh pickling liquor is
continuously added. In order to prevent the nanofiltration plant from blocking
by particles a microfiltration pre-treatment was established.
The nanofiltration plant is specified as follows (Tab. 4):
Table 4. Specifications
of the nanofiltration membrane plant [12]
|
L*W*H |
4000*1900*2100
mm |
|
Materials |
PE,
Hastelloy C22, Teflon coated tubes |
|
T
(max) |
30 °C |
|
p
(max) |
40 bar |
|
Feed
(max) |
1.7 m³/h |
|
Membrane |
6
Spiral wound module (22 m²/module) |
A picture of the nanofiltration plant is shown
below (Fig. 5)

Figure 5. Nanofiltration membrane plant for
the filtration of nitric/fluoric acid pickling.
A quantity of 1.7 m³/h of pickling liquor with
a mean concentration of 35 g/l metal, 50 g/l fluoric acid and
110 g/l was flowing into the membrane plant and concentrated up to
1.25 VCF.
As result, it succeeded in recovering
15.6 kg/h fluoric acid and 60.0 kg/h nitric acid. Depending on the
prices of pickling liquor and the life time of the membranes a pay-pack period
of maximum 5 years was calculated [12].
V.
CONCLUSIONS
Particularly small and medium enterprises in
the sector of metal finishing are sceptic towards CP methods since they fear
high investment costs. However, already very simple measures like installations of drip trays and splash guards
contribute to reasonable saving of waste and material.
In the long run the
integration of advanced but more elaborate CP processes, e. g. membrane filtration, for closing process
water loops sustainably improve the efficiency of existing processes and helps
to reduce the amount of waste-water. One main advantage of membrane processes
is the fact that there is no interference with the original process chain,
since membrane applications are an additional modular operation step.
They are to be
operated both continuously or batch wise, are place-saving and, with regard to
pickling recycling, have significant lower energy consumption.
However, further research
activities have to be conducted in order to increase the reliability of the
membrane process and the lifetime of the membrane at strong acidic conditions.
REFERENCES
1. Brown C. J., 2002. Recovery of stainless steel pickle
liquors purification vs. regeneration. CISA
Intern. Steel Congr.,
2. Centre for Excellence in Cleaner Production,
2002. Waste
minimization in metal surface Ffinishing.
3. Cleaner Production International LLC, 2009. The metal products industry,
http://www.cleanerproduction.com, 08/2009.
4. Environmental Guidelines for small-scale
activities in Africa, 2006. Micro- and small enterprises – Metal finishing.
5. EPA, 1993. Guides to pollution prevention - The metal
finishing industry. 10/1992.
6. German Industrial Bank, 2009. Metal Industry 2020.
7. Kleemann M., 2001. Aufbereitung einer Abfallphosphorsäure und einer Fluss-/Salpeter-Abfallmischsäure mittels Membrantechnik. Master thesis, Fraunhofer UMSICHT.
8. Koefoed M., C. Buckley, 2008. Clean technology transfer: A case
study from the South African metal finishing industry, 2000-2005. J. of Cleaner Production, 16 : 78-84.
9. Lyko M., 2005.
Zu sauer? – Nicht für Membranen. Chemie Ingenieur Technik, 77/5 : 598-592.
10. Reeve D.J., 2007. Environmental improvements in the metal
finishing industry in
11. Rituper R., 1993. Beizen von Metallen. Leuze Verlag.
12. Siegert K.,
2004. Untersuchungen zur Wiederverwendung von
Abwasserströmen aus der Oberflächenbehandlung in der Stahlindustrie mittels
Membrantechnik. Student research project,
Fraunhofer UMSICHT.
13. Telukdarie A., C. Buckley, M. Koefoed, 2006. The importance of assessment tools
in promoting cleaner production in the metal finishing industry. J. of Cleaner Production, 14 : 1612-1621.
14. UNEP Working Group for Cleaner Production,
1998. A cleaner production manual for the
metal finishing industry. CRC for Waste
minimization and Pollution Control, Ltd.